Optimal Typeface and Type Size on Thai Drug Labeling and Drug Documentation: A Recommendation for Legal Development
Abstract
Background An earlier study by Punsongserm and Suvakunta (2022) investigated how using proper typefaces and type sizes on Thai drug packages and patient information leaflets affects reading times and participants’ satisfaction. The study was an initial review of the use of Thai typefaces focusing on typeface classifications and type sizes. The study also conducted a pilot study with participants who read small sample text cards that varied by typeface and type size. To extend the results of the previous study, this current study is a set.
Methods First, we conducted a literature review about guidelines and regulations governing typefaces and type sizes on drug labeling and drug documentation, followed by an investigation of word accuracy identification in reading Thai transliterated words on drug documentation. Later, we measured user preference in different typefaces and type sizes through user manuals of the SARS-CoV-2 Antigen Test Kit (ATK) with and without wearing cataract-experiencing goggles.
Results The conventional text typefaces with distinctive key letter features have a lower misreading in word accuracy. Also, the most satisfaction in the user preference test importantly indicate a user manual that provides a conventional text font with a larger type size. The international guidelines recommend a minimum type size of 6 points or 1.4 mm of x-height. In contrast, Thailand’s national regulation suggests a minimum size between a Bo Baimai height of 1–2 mm for food labels and more than 2 mm for drug products. However, we recommend that a Thai type size may be 1.3–2 mm of Bo Baimai height for reading body text, and the type size for headlines and subheads should be more remarkable. The use of smaller type sizes needs a case-by-case evaluation basis based on legibility and readability.
Conclusions The present study examines how using proper typefaces and type sizes on Thai drug packages, patient information leaflets, and medical products affects reading accuracy and participants’ satisfaction. The study suggests that using illegible typeface and very small type size on many Thai drug packages, patient information leaflets, and medical products may be inappropriate and not serve a variety of readers. Therefore, the review and enforcement of the law should be considered in conjunction with developing guidelines and regulations based on user-centered care.
Keywords:
Accessible Typography, User-Centered Information Design, Thai Typeface, Type Size, Text Legibility, Usability, Drug Labels and Documentation, Recommendation for Legal Development1. Introduction
The text legibility of drug labeling and documentation is vital for drug security and practical use after medicines are prescribed or dispensed in drugstores or hospitals. Patients or users may independently use the drug packages or patient information leaflets to answer their questions, such as information on indications, dosage, precautions, and drug interactions.
Patient-centered care is a principle of care that considers the user’s experience, values, needs, and preferences in the planning, coordinating, and delivery of care (Gluyas, 2015). These are professional obligations to take responsibility for the individual needs of the patient or user, including ensuring patients comprehend their medications and how to use them; one issue that has gained minor attention is the legibility of drug labels (Leat et al., 2014).
According to Punsongserm and Suvakunta (2022) examined how using appropriate typefaces and type sizes on Thai drug labeling and drug documentation affects reading times and participants’ satisfaction. Punsongserm and Suvakunta also revealed that minor regulations or guidelines had been developed and implemented, reflecting that the designers and medicine entrepreneurs lack adequate knowledge and social responsibility, based on optimistic typography. Therefore, these findings suggest the necessity for much further research.
The current study aimed to continue investigating aspects of optimal type sizes and proper typefaces that can facilitate a variety of readers in small text sizes for Thai drug packages and patient information leaflets. This study intends to instigate improvements in the Thai regulations and guidelines for drug labeling and drug documentation, focusing on selecting an appropriate typeface and determining type size.
2. Method
The methods in the present study include three stages. 1) The literature review concerns the guidelines and regulations for typefaces and type sizes on drug labeling/drug documentation. 2) We investigate word accuracy identification in reading the Thai transliterated words on drug documentation. 3) We measure user preference for the user manuals of the SARS-CoV-2 Antigen Test Kit (ATK).
2. 1. Literature Review of Guidelines and Regulations
This stage was a literature review through a survey of significant guidelines and regulations focused on using typefaces and type sizes in drug labeling and drug documentation in advanced developing countries, emphasizing typography in drug labeling and drug documentation.
2. 2. Investigation of Word Accuracy Identification
In the previous study, we tested the reading of the small text sizes in different typefaces through participants’ reading of sample texts of Thai drug content to measure the comparative time that readers spent (Punsongserm & Suvakunta, 2022). The finding suggested that the conventional Thai text fonts facilitated the most readers more than the Roman-like Thai fonts. To examine the capability of typefaces in small type sizes, in the present report, we reconsidered analyzing the data collected in the previous study on the accuracy of word identification focused on transliterated words. Reading transliterated words set in small text can reveal the advantages and disadvantages of using different typefaces. Because the transliterated words in drug terminology are unfamiliar, the reader needs to spell them rather than simply recall them from word recognition and memory. Table 1 shows the selected 14 Thai transliterated words used in the sample text of drug documentation as test materials.
We listened to the recordings of participants’ voices reading sample texts of drug content from Punsongserm and Suvakunta (2022) and checked them for reading accuracy of the 14 Thai transliterated words.
2. 3. User Preference Test
1) Selected User Manuals
To measure the performance of typefaces using various type sizes, we employed the four user manuals for the SARS-CoV-2 Antigen Test Kit as a medical product, which are widely available in the market and at drugstores. Each user manual was typed in different typefaces, type sizes, and typographic design (e.g., line spacing, alignment, and line length). The kind of printing paper for the user manuals was uncoated paper. We measured the type sizes displayed on each user manual in millimeters by a digital microscope (KUAIQU: 180X lens magnification with 38MP FHD Camera V6). This method enabled us to observe a much more significant character size, as measured and captured via a Ultra High Definition Monitor (BenQ BL2711U 27 inch). Table 2 and Figure 1 exhibit the typographic specifications, type sizes, and partial typefaces of the four selected user manuals.

Typographic Specifications of User Manuals for the SARS-CoV-2 Antigen Test Kit, Brands A, B, C, and D

Approximately Type Sizes Used on the User Manuals for the SARS-CoV-2 Antigen Test Kit, Brands A, B, C, and D
2) Apparatus
We used the three reading lamps that can adjust the brightness level. We set each lamp’s illuminance for 100 lux (first lamp), 200 lux (second lamp), and 300 lux (third lamp). The illuminance levels were measured with a TENMARS TM-209M MULTI-LED Light Meter. We installed the reading lamps on a table sorted from left to right as follows: first lamp (100 lux), second lamp (200 lux), and third lamp (300 lux). The distance of each lamp was approximately 35 cm from the edge of the lampshade to the tabletop.
In the current study, we applied cataract simulation goggles (Panasonic Corporation, 2021) called cataract-experiencing goggles (Obama et al., 2005; Wongsompipatana et al., 2011; Waleetorncheepsawat et al., 2013; Rattanakasamsuk, 2013) to simulate elderly vision and low visual acuity. The property of the goggles was Snellen acuity of 6/15 (Phuangsuwan & Ikeda, 2017), which is equivalent to LogMAR 0.4.
A sample of 21 native Thai volunteers with normal or low visual acuity no more than logMAR 0.2 (average = logMAR 0.052), including eight males and 13 females between 18 and 62 years old (average = 39 years) participated in this study. Table 3 shows each participant’s age, gender, educational background, occupation, and visual acuity.
We experimented in a controlled dark room in a laboratory. We conducted data collection in a well-ventilated location. Before the user preference test, we applied a mobile application called Peek Acuity (Bastawrous et al., 2015; Kawamoto et al., 2021) to measure the visual acuity of each participant.
The experiment was conducted with two tasks: with and without cataract-experiencing goggles. We asked participants to wear the cataract-experiencing goggles in the first task but not in the second. When a participant was ready for the first task and wearing the cataract-experiencing goggles, we randomly chose a SARS-CoV-2 Antigen Test Kit user manual out of the four user manuals. We presented the chosen user manual on a tabletop to the participant under the center of the brightness of the first lamp (100 lux), followed by the second lamp (200 lux), and finally, the third lamp (300 lux). At each step of the presentation, with the different illuminance levels, we randomly selected partial texts from the user manual and asked a participant to read them aloud. The reading distance from the participant to the user manual was approximately 35 cm. We randomly selected a second user manual when the participant finished reading their first chosen user manual. We continued until the participant had read all four user manuals. We conducted the second task the same as the first, but the participant did not wear the cataract-experiencing goggles. For both tasks, when finished, we asked the participant to do a rating-scales questionnaire that used a Likert-type scale with five response points (Likert scales, System Usability Scale [SUS]) (Rosala, 2020). The five rating scales consisted of 1 (very difficult), 2 (difficult), 3 (neither easy nor difficult), 4 (easy), and 5 (very easy). Later, we also asked the participant to sort the user manuals, based on their satisfaction and ease of reading, from easiest to most difficult.
3. Result
3. 1. Guidelines and Regulations on Drug Labeling and Drug Documentation: Typeface and Type Size
Most labeling guidelines and regulations did not specify a specific typeface. They suggest choosing a legible typeface that is easy to read for consumers (European Commission, 2009; Government of Canada, 1999; IC Optix, 2015). However, specific guidelines recommend the use of a sans serif type style (e.g., Helvetica, Univers, and Arial; Institute for Safe Medication Practices Canada, 2018, 2019; National Patient Safety Agency, 2007; Her Majesty the Queen in Right of Canada, 2016). In addition, using a sans serif typeface must not be compressed, expanded, or decorative (Institute for Safe Medication Practices Canada, 2018, 2019; Her Majesty the Queen in Right of Canada, 2016), and it must use bold or semibold, avoid lightweight typestyles, and not use a condensed typeface (National Patient Safety Agency, 2007).
The Guideline on the Readability of the Labeling and Package Leaflets of Medicinal Products for Human Use recommends that a minimum type size for labeling and package leaflets should be 9 points, as measured in Times New Roman font (European Commission, 2009). Conversely, the Guide to Labels and Leaflets of Human Medicines suggests a minimum of 9 points for the package leaflet and 7 points for labels (Health Products Regulatory Authority, 2022). We converted the point sizes of 7- and 9-points Times New Roman to millimeters; 7 points has a cap height of 1.635 mm and x-height of 1.104 mm, whereas 9 points has a cap height of 2.102 mm and x-height of 1.420 mm (see Table 4). The Good Label and Package Practices Guide for Non-prescription Drugs and Natural Health Products determines that a type size should be used that can facilitate a variety of users, and the largest type size should be provided as much as possible. Key information on labels and packages should not use a type size of less than 6 points (Institute for Safe Medication Practices Canada, 2018, 2019). For tiny prescription products, the type size should be at least 1.5 mm high (Institute for Safe Medication Practices Canada, 2019). The Guidance for Industry: Labeling for Human Prescription Drug and Biological Products—Implementing the PLR Content and Format Requirements recommends the type size requirements for Food and Drug Administration (FDA)-approved patient labeling be a minimum of 6–10 points for trade labeling (i.e., labeling on or within the package from which the drug is to be dispensed) and other labelings (e.g., labeling accompanying promotional materials; U.S. Department of Health and Human Services, 2013). The Design for Patient Safety: A Guide to the Graphic Design of Medication Packaging has determined that the minimum font size should be 12 points and provides a minimum of 14 points for patients with sight difficulties (National Patient Safety Agency, 2007). The guidance document packaging for human medicinal products HMV4 requires a minimum size of the cap height as 1.4 mm or 6 points (Swissmedic, 2022). The Labelling of Medicinal Products by Federal Agency for Medicines and Health Products assigns that at least 7 points or a minimum x-height of 1.4 mm should be used on the label of all medical products (Federal Agency for Medicines and Health Products, 2020).
Furthermore, the Guide to the Consumer Packaging and Labeling Act and Regulations defines that the cap height of type (uppercase) must be a minimum of 1.6 mm. When using only lower case; the minimum size must be a 1.6 mm x-height. When using type on a principal display surface of 10 square centimeters or less, the minimum type height may be reduced to 0.8 mm (Government of Canada, 1999). In addition, the Guidance for Industry Labeling Over-the-Counter (OTC) Human Drug Products recommends the type sizes in labeling formats (standard format) should be no smaller than 8 points for drug facts, with at least 8 points or more than 2 points sizes of text added for headings, and no smaller than 6 points for subheadings and bulleted text (U.S. Department of Health and Human Services, 2018).
The survey of guidelines and regulations suggested using various typefaces and type sizes on drug labeling and drug documentation. To know the actual sizes of each point size, we converted the selected point sizes (e.g., 6, 7, 8, 9, 12, and 14 points) to physical sizes in millimeters for Times New Roman, Arial, Helvetica, and Univers, as shown in Table 4.
For the regulations in Thailand, we found that Article 25 of the Drug Act (No. 5), B.E. 2530 (A.D. 1987) specifies that “the texts on the label and medication leaflet must be able to be read clearly” (Office of the Council of State, 2019, p. 4). Furthermore, the Announcement of the Ministry of Public Health (No. 383) B.E. 2560 (2017) about the regulations on the type sizes on Thai labels, “Subject: Displaying Food Labels on the Packages (No. 2),” defines the requirements for the text sizes for food quantity, key ingredients, allergen information, and expiration dates be no smaller than 1, 1.5, or 2 mm, depending on the label area size of each product (Government Gazette, n.d.).
Accordingly, the Guideline for the Development of Patient Information Leaflet (Food and Drug Administration, 2013, 2019) in the Guideline for Leaflet Development issued by the Food and Drug Administration has suggested that the drug documentation attached to a drug product should display information with a Thai typeface no smaller than 11-points Tahoma for the subheading; subheading content; footer text; and information about the manufacturer, importer, and distributor. Also, a font no smaller than 14-points Tahoma should be used at the beginning of a document (drug name, strength, dosage form, and trade name) and the heading. As measured, the Bo Baimai height of 11-points and 14-points Tahoma were 2.118 mm and 2.691 mm, respectively.
3. 2. Word Accuracy Identification
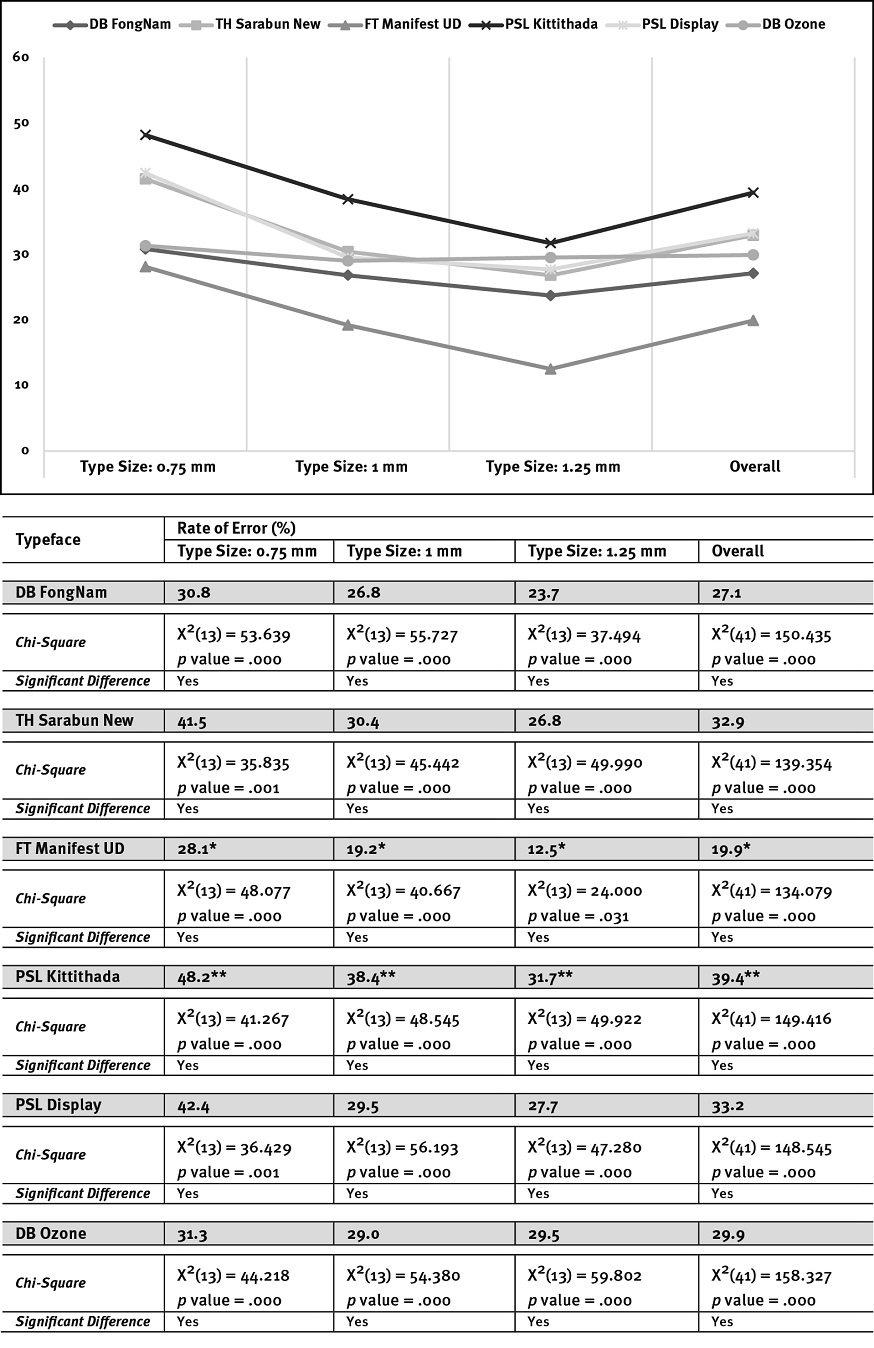
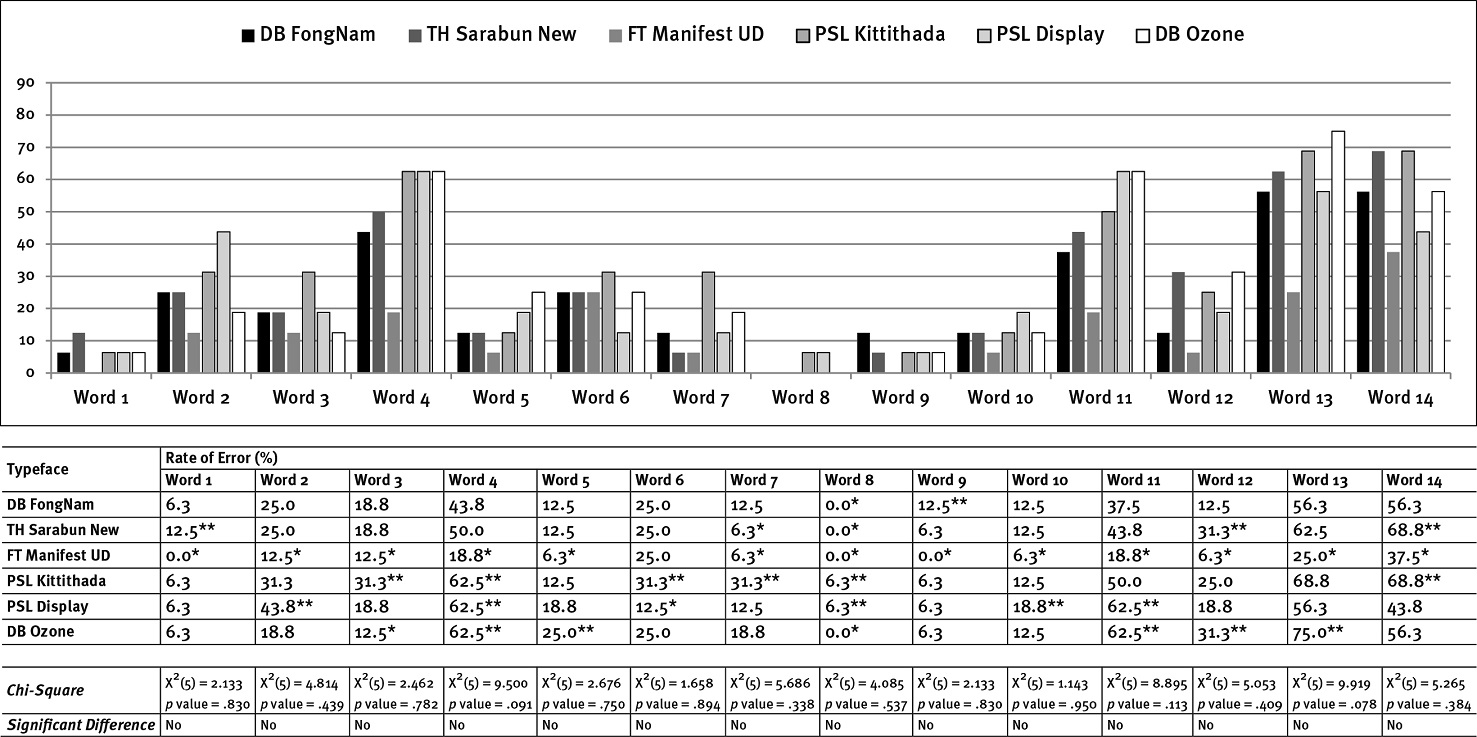
The findings suggested that the errors in word accuracy identification of the text displayed at each smaller font size (0.75 mm) have more significance than the other font sizes (e.g., 1 mm and 1.25 mm; see Figure 2). It showed that findings of a type size of 0.75 mm, PSL Kittithada, has the highest likelihood of misreading at 48.2%, followed by PSL Display (42.4%), TH Sarabun New (41.5%), DB Ozone (31.3%), and DB FongNam (30.8%). In comparison, FT Manifest UD has the lowest rate of error at 28.1%.
The type size of 1 mm findings showed that each typeface’s errors decreased; however, the highest misreading was still apparent in PSL Kittithada, with a 38.4% rate of error. Surprisingly, TH Sarabun New received a percentage of error (30.4%) similar to PSL Display (29.5%) and DB Ozone (29%). DB FongNam had a lower misreading at 26.8%, and FT Manifest UD had the lowest error at 19.2% (see Figure 2).
The findings of the larger type size of 1.25 mm suggested that the misreading rate of most typefaces decreased from the results of other font sizes except the finding of DB Ozone that still had a similar percentage of error, with 29% for type size 1 mm and 29.5% for type size 1.25 mm. The most errors were still apparent in PSL Kittithada at 31.7%, whereas FT Manifest UD had the lowest error at 12.5% (see Figure 2).
Overall, the findings revealed that Roman-like Thai fonts, such as PSL Kittithada, had the highest misreading at 39.4%, followed by PSL Display (33.2%). Thai conventional text fonts, such as FT Manifest UD, received the lowest rate of error at 19.9%, followed by DB FongNam (27.1%). The results suggested that conventional Thai text fonts can enhance word accuracy identification better than Roman-like Thai fonts. Surprisingly, the findings of TH Sarabun New (Thai conventional text font) showed quite a high rate of error, similar to PSL Display (the Roman-like Thai font), in results of type sizes 0.75, 1, 1.25, and overall (see Figure 2).
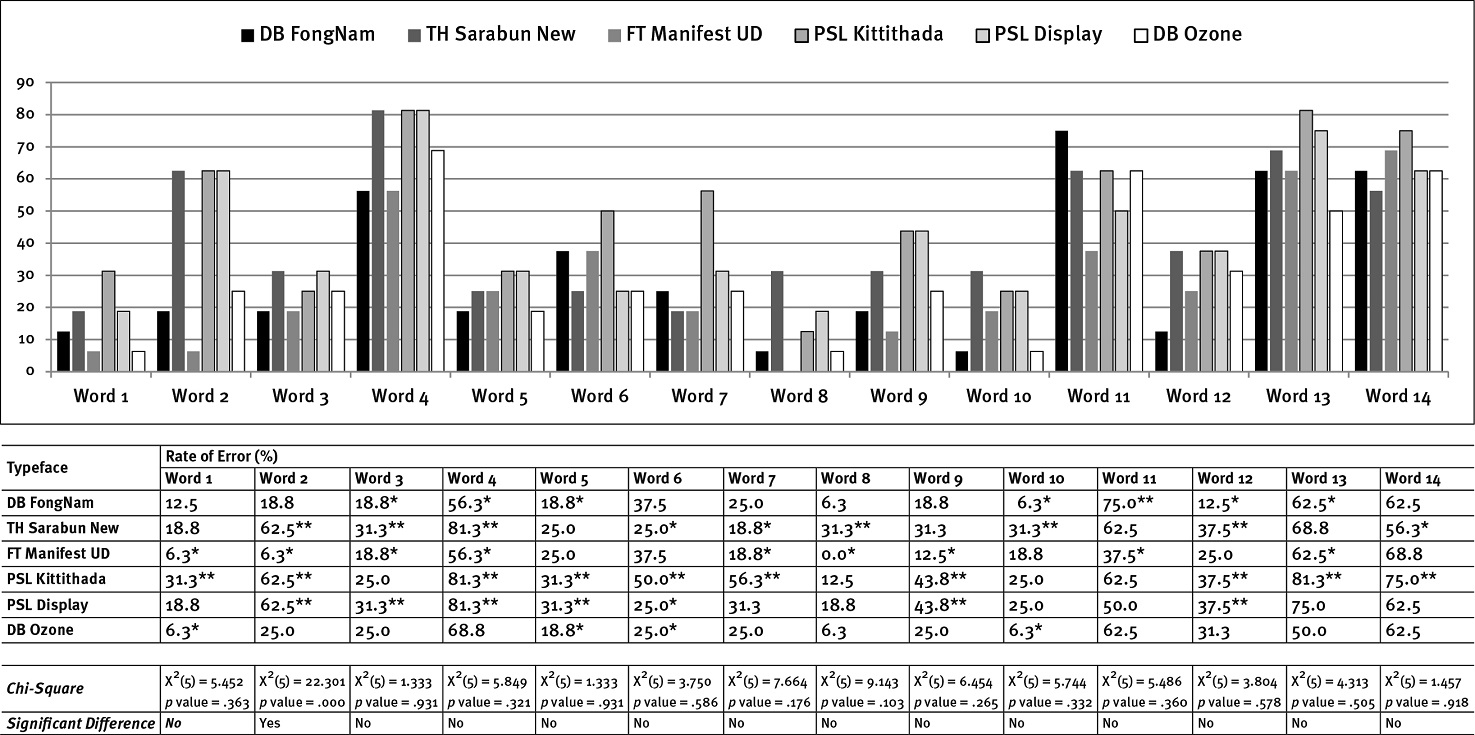
Referring to Table 1, Thai Transliterated Words, Figure 3 exhibits errors in word accuracy identification compared to words of type size 0.75 mm. In the case of the Roman-like Thai font, PSL Kittithada showed the most significant misreading in words 1, 2, 4, 5, 6, 7, 9, 12, 13, and 14. In addition, PSL Display illustrated the most significant misreading of words 1, 3, 4, 5, 9, and 12. However, PSL Display had the lowest error for word 6; in addition, DB Ozone obtained the lowest misreading for words 1, 5, 6, and 10. In the case of the Thai conventional text fonts, FT Manifest UD received the lowest misreading for words 1, 2, 4, 5, 6, 7, 9, 12, 13, and 14; likewise, DB FongNam obtained the lowest error in words 3, 4, 5, 10, 11, 12, and 13. In contrast, TH Sarabun New had the highest misreading in words 2, 3, 4, 8, 10, and 12.
When reading words in font size 1 mm, most errors decreased when compared to the type size 0.75 mm. The words that had the lowest rate of misreading were word 1 (สเตอรอยด์) and word 8 (ดารุนาเว ียร์; see Figure 4). The most misreading occurred in the findings of the Roman-like Thai typefaces, PSL Kittithada (word 3, 5, 6, 7, 8, 11, 12, and 13) and PSL Display (word 1, 2, 4, 7, and 11); in addition, TH Sarabun New (Thai conventional text fonts) showed the highest error in words 3, 8, 9, and 10 (see Figure 4). Conversely, FT Manifest UD had the lowest rate of misreading for words 1, 2, 3, 4, 5, 7, 11, and 13. Similarly, DB FongNam obtained the lowest error in words 3, 5, 8 (no error), and 12; PSL Display exhibited the lowest error for words 9, 10 (no error), 12, and 14, and DB Ozone did for words 1 (no error), 3, 6, and 9.
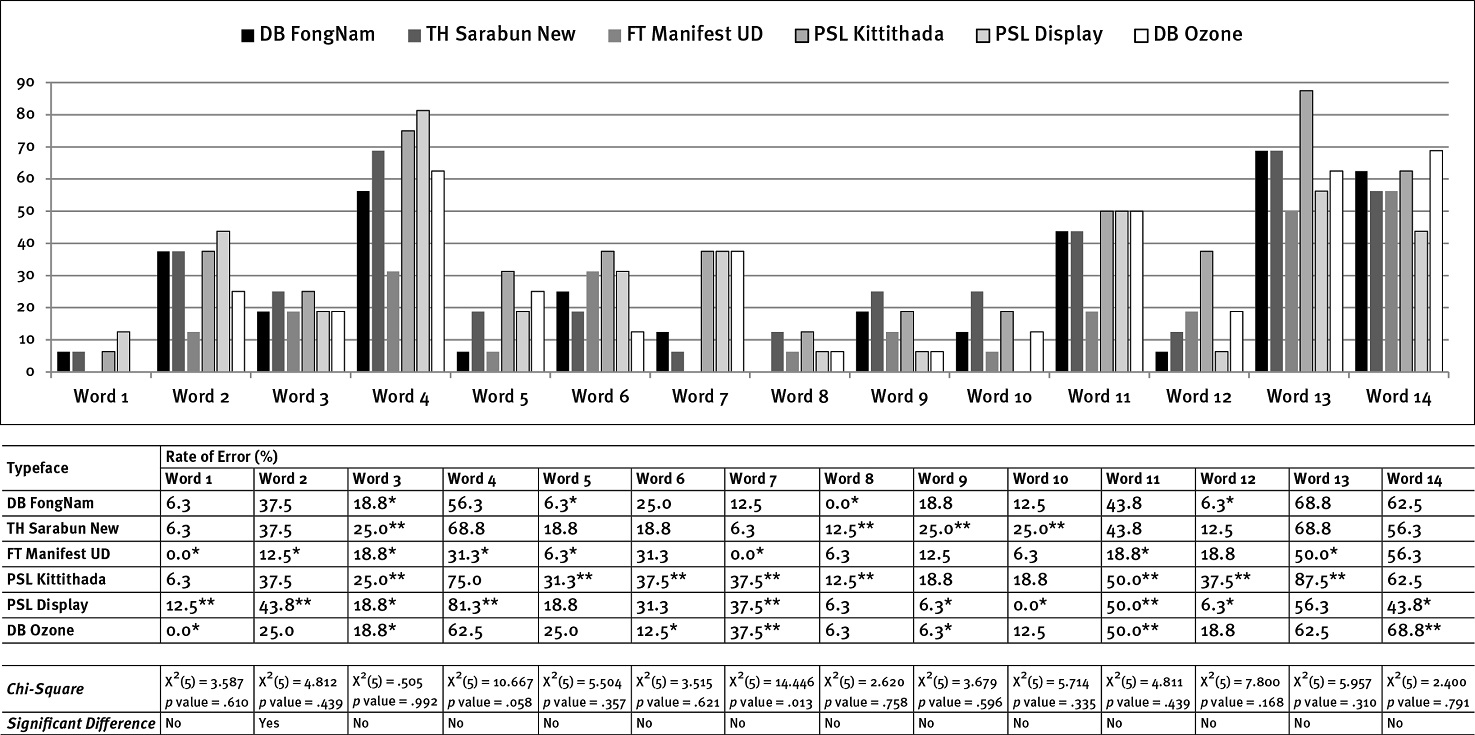
The findings In Figure 5, type size 1.25 mm, illustrated that most of the results of FT Manifest UD had the lowest rate of errors, except in word 6. PSL Kittithada still had a higher rate of misreading than the other typefaces, particularly in words 3, 4, 6, 7, 8, and 14. Besides, the findings of PSL Display showed the highest misidentification in words 2, 4, 8, 10, and 11 (significantly in words 2, 4, and 11). In addition, the highest rate of errors occurred in DB Ozone, e.g., words 4, 5, 11, 12, and 13 (more evidently in words 4, 11, and 13) but had the lowest misreading rate for the words 3 and 8 (no error).
The words that had high error rates in most typefaces and type sizes (0.75, 1, and 1.25 mm) included words 4 (โปรต ีเอสอ ินฮ ิบ ิเตอร์), 11 (อ ีร ีโทรไมซ ิน), 13 (โทรล ีแอนโดรไมซ ิน), and 14 (รูมาทอยด์), On the contrary, the words with the lowest misreading rates in most typefaces and type sizes were words 1 (สเตอรอยด์), 8 (ดารุนาเว ียร์), 9 (ซาคว ินาเว ียร์), and 10 (มาโครไลด์), especially in type sizes 1 and 1.25 mm (see Figures 3, 4, and 5).
3. 3. User Preference Test
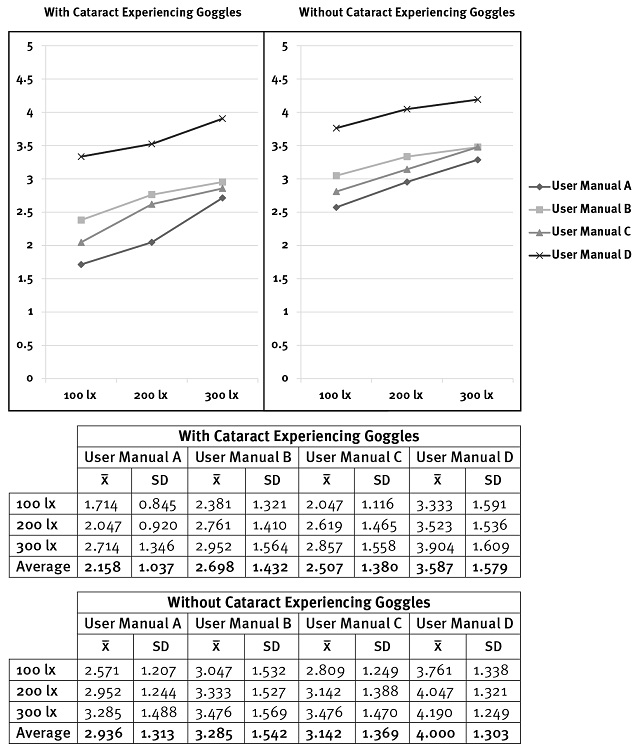
Figure 6 illustrates the comparative findings of the user preference tests for the four user manuals for the SARS-CoV-2 Antigen Test Kit with and without cataract-experiencing goggles. The simulation findings for cataract vision (with logMAR 0.4) indicated that the User Manual D (typed with a conventional Thai text font) obtained an average satisfaction of rating-scale 3.587. User Manual D had a satisfaction rating of 3.333 for illumination 100 lux, 3.523 for 200 lux, and 3.904 for 300 lux (see Figure 6). This indicates that User Manual D is quite easy to read. In contrast, the results of User Manuals A, B, and C received relatively low satisfaction, especially in User Manual A (typed with a Roman-like Thai font), with an average satisfaction rating of 2.158 (quite difficult), especially low in the result of 100 lux (1.174; see Figure 6). Although the text of User Manual C was typed with a conventional Thai text typeface, it had relatively low satisfaction (average satisfaction = 2.507). It was slightly lower than the results of User Manual B, typed with a Roman-like Thai typeface and with an average satisfaction of 2.698 (see Figure 6).
With an average logMAR of 0.052 (without cataract-experiencing goggles), the overall findings revealed that satisfaction increased over the simulation findings for cataract vision. User Manual D still obtained the most satisfaction compared to the other user manuals, with an average satisfaction of rating-scale 4 (easy to read), including 3.761 for illumination 100 lux, 4.047 for 200 lux, and 4.190 for 300 lux (see Figure 6). User Manual B received a slightly higher average satisfaction compared to User Manual C (3.285 and 3.142, respectively). Moreover, User Manual A received the lowest average satisfaction, at 2.936 (nearly rating of neither-easy-nor-difficult—moderate; see Figure 6).
According to the procedure, upon completing the user manual reading task, we asked each participant to sort the user manuals based on their satisfaction, from most to least satisfied. Table 5 shows that most participants assigned rank 1 for satisfaction to User Manual D, with 20 scores (95.23%). Meanwhile, participants largely assigned rank 2 to User Manual B, with 13 scores (61.90%), followed by User Manual C for rank 3, with 11 scores (52.38%). In comparison, participants assigned rank 4 to User Manual A, with 15 scores (71.42%).
The results of sorting user manuals coincided with satisfaction on the rating scales results. In other words, most participants (20) assigned User Manual D as rank 1, corresponding to the average satisfaction of 3.587 and 4 (with and without cataract-experiencing goggles, respectively). Besides, 13 participants assigned rank 2 to User Manual B, corresponding to an average satisfaction of 2.698 (with cataract experiencing goggles) and 3.285 (without cataract experiencing goggles), followed by User Manual C as rank 3, corresponding to an average satisfaction of 2.507 (with cataract experiencing goggles) and 3.142 (without cataract experiencing goggles) (see Figure 6). Furthermore, 15 participants assigned rank 4 to User Manual A, corresponding to an average satisfaction of 2.158 (with cataract experiencing goggles) and 2.936 (without cataract experiencing goggles) (see Figure 6).
4. Discussion
4. 1. Typeface
Typeface is an essential factor and should be the foremost consideration when deciding how to best convey information in drug labeling and documentation. It is an important variable related to the design of letterforms concerning type legibility and type personality. The literature suggests that conventional text typeface with a superior Roman-like Thai typeface generates confidence in users and readers. However, those selecting a conventional text typeface for use in a small size should consider typefaces with distinctive letter features that facilitate reading in a small text size. FT Manifest UD typeface is an example of a design with distinctive letter features, broad counter space, jutting parts, and visible small letter features (Punsongserm, 2019, 2020) (see Figure 7). Even though TH Sarabun New is a conventional text typeface, our current study’s results revealed that more errors than the other conventional text typefaces occurred in the word accuracy identification task, and the studies by Punsongserm (2019, 2020) indicated that TH Sarabun is at a disadvantage in low visual acuity conditions. This suggests that TH Sarabun New may have insufficient distinctive key letter features to facilitate reading in a small text size.
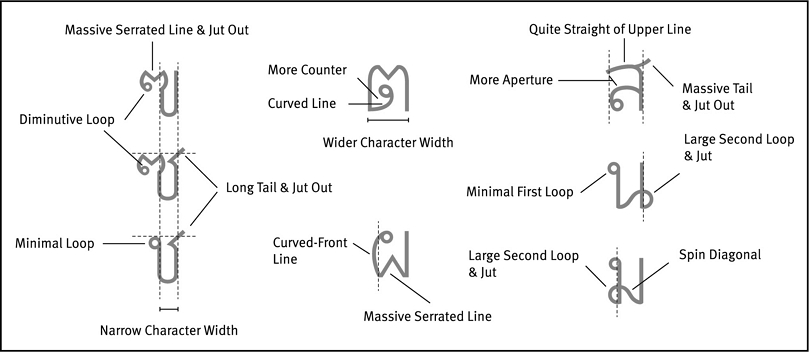
Examples of Key Letter Features in FT Manifest UD Typeface, Prototype of Thai UD Typeface(Source: Punsongserm et al., 2017b: 43; Punsongserm et al., 2018a: 2; Punsongserm et al., 2018b: 112)
There is evidence that greater boldness, or visual angles rather than regular boldness, in medium size for Roman letterforms can contribute to legibility in smaller type sizes; however, extreme letter boldness does not enhance recognition (Beier & Oderkerk, 2019). Additionally, the effect of character width in ultra condensed font does not facilitate eye movements during reading (e.g., fixations and saccades), while extended font provides positive effects on eye movements (Minakata & Beier, 2021). These results reinforce the National Patient Safety Agency’s recommendation to use proper bold typeface and avoid condensed typeface in drug labeling and documentation (2007). In addition, broader fonts improve recognition and accurate reading compared to narrower fonts in parafovea and periphery (Oderkerk & Beier, 2022). Moreover, increased letter spacing, letter width, and letter boldness enhance performance in low-vision reading owing to age-related macular degeneration (AMD) (Beier et al., 2021). This knowledge could be applied to Thai typography as a universal principle because it concerns visibility (e.g., positive and negative space) rather than recognition in language. We found no studies investigating the effectiveness of thickness and broadness in Thai typefaces, particularly in small type sizes. This deficiency is an opportunity for further study.
It is well-known among typographic designers that the proportion of letterforms (e.g., horizontal and vertical scales) affects text legibility. Excessive variance in the horizontal or vertical scale can distort the letterforms to the point that texts or words become illegible, such as when desktop publishing programs make the horizontal scale too tight or loose or the vertical scale too small or large (Wilkinson, 2005). Specific guidelines suggest avoiding narrowed typefaces (European Commission, 2009; Health Products Regulatory Authority, 2022; Her Majesty the Queen in Right of Canada, 2016; Institute for Safe Medication Practices Canada, 2018, 2019) and compressed or condensed typefaces (Her Majesty the Queen in Right of Canada, 2016; Institute for Safe Medication Practices Canada, 2018, 2019; National Patient Safety Agency, 2007). The Design for Patient Safety: A Guide to the Graphic Design of Medication Packaging suggests using bold or semi bold types and avoiding lightweight types (National Patient Safety Agency, 2007). These recommendations can also improve typeface selection in Thai drug packages or patient information leaflets as general rules for typographic design.
The sans serif typefaces have no decorative extensions. They appear minimalist and clean and are larger than the serif typefaces at the same point size (Her Majesty the Queen in Right of Canada, 2016; Institute for Safe Medication Practices Canada, 2018). However, these differences cannot be correlated when comparing Thai text typeface and Roman-like Thai typeface. Serif font may include decorative parts of each Roman letterform that do not help differentiate between letters or enhance legibility, except in the case of the uppercase “I” (Punsongserm et al., 2018c). In contrast, the seemingly decorative loops and key letter features of Thai characters are not decorative; rather, they are essential aspects that improve readability (Punsongserm et al., 2018c). In addition, the glyphs of Roman-like Thai typefaces were patterned on and adapted from the original Roman or Latin letterforms (which are sans serif); therefore, the key features have been either simplified or omitted (Punsongserm et al., 2018c).
Mitchell (2014) stated that Roman-like Thai types have fewer feature details, suggesting they improve visibility at a small type size. Notwithstanding, the legibility of types at small sizes may become inefficient and make meaning less precise because “less detail” means diminishing or omitting key features of Thai letterforms (Punsongserm et al., 2018c). An advantage of the Roman-like Thai fonts is their increased Bo Baimai height compared to conventional Thai fonts; this enables Roman-like Thai fonts to have more significant consonants than conventional Thai fonts at the same point size (Usakunwathana, 2015). However, omitting the loops and some features in the Roman-like Thai typeface causes cramped letter spaces, which are affected by the lack of loop representatives and jutting parts (Punsongserm & Suvakunta, 2022).
We do not recommend using a Roman-like Thai typeface because in our study we found that Roman-like Thai typeface, especially PSL Kittithada, was more often misread than the conventional text typefaces during the word accuracy identification task. The Antigen Test Kit (ATK) user manuals typed with the Roman-like Thai typefaces also generated little user satisfaction in the user preference test. Moreover, in the previous study, using Roman-like Thai typefaces inferiorly affected participants’ reading time (Punsongserm & Suvakunta, 2022). However, DB Ozone (Roman-like Thai typeface) outperformed the other Roman-like Thai typefaces in our study’s word accuracy identification task and outperformed some Roman-like Thai typefaces in the reading time test of Punsongserm and Suvakunta (2022). This previous study (Punsongserm & Suvakunta, 2022) suggested that DB Ozone has loop representatives (short horizontal lines), which broaden letter spaces better than loopless ones.
Accordingly, determining Thai typeface classification for use in small size on drug labeling and documentation and medical products should specify details regarding distinctive key letter features that enhance legibility and should provide examples of good typefaces. Roman-like Thai typefaces might be allowed in specific cases when they are carefully considered and include jutting parts and loop representatives, as well as appropriate letter spacing when displayed in small type size. Importantly, these must be used with large types or for headlines and subheads, exceeding the recommended size of conventional text fonts.
4. 2. Type Size
Most international guidelines and regulations for drug labeling and documentation recommend a minimum type size for x-height. X-height should be at least 1 mm (6 points) (see Table 4). In contrast, Thailand’s guidelines and regulations suggest a minimum size of 1–2 mm for food labels (Government Gazette, n.d.) and a minimum of 2.118 to 2.691 mm (11–14 points) for drug products, as measured in Tahoma (Food and Drug Administration, 2013; Food and Drug Administration, 2019; Punsongserm & Suvakunta, 2022). If we assume the reading distance is 35 cm, the type sizes of 1, 2, 2.118, and 2.691 mm correspond to the visual angles of 0.1637°, 0.3274°, 0.3467°, and 0.4405°, respectively.
This study elucidates how differences in type size in words and text for drug labeling and documentation and medical products affect legibility and ease of reading. Punsongserm and Suvakunta (2022) also clarified that the difference in type size affects the time spent reading. We found several examples of small text size used in drug labeling and documentation and medical products. The smallest type size was 0.5 mm Bo Baimai height (visual angle of 0.0819° at a viewing distance of 35 cm) (see Table 2 and Figure 1). This does not conform to Thai guidelines and regulations on minimum type size.
Although international guidelines and regulations recommend a minimum type size of 6 points. or 1.4 mm for x-height (see Table 4), this size cannot be universally applied to the use of Thai typeface on drug labeling and documentation. Thai characters have more complex letter features than Roman characters and are more easily confused in certain pairs (Punsongserm et al., 2017a, 2017b). Consequently, Thai text on drug labeling and documentation on medical products may need a minimum size larger than recommended by international guidelines to accommodate a variety of readers, such as older adults and the visually impaired.
The results of the current study’s user preference test suggest that the ATK User Manual-Brand D generated the highest user satisfaction. The type sizes of body text used were 1.5 and 1.7 mm at a distance of 35 cm, which correspond to the visual angles of 0.2456° and 0.2783°, respectively. In comparison, the other ATK User Manuals had visual angles of 0.1310° (0.8 mm), 0.1473° (0.9 mm), and 0.1637° (1 mm). Also, the results of our word accuracy identification test reveal that use of type size 1.25 mm resulted in fewer reading errors than other type sizes. This type size corresponds to the visual angles of 0.2046° at a reading distance of 35 cm. Regarding type sizes that are easy to read, Santayayon et al. (2011) suggested that the minimum type sizes for both young and older adults should be 2 mm at a viewing distance of 50 cm. This recommended type size corresponds to the visual angle of 0.2292°, which is similar to the angles of both the type size of body text used in ATK User Manual-Brand D (0.2456° and 0.2783°) and the type size of 1.25 mm used in our test of word accuracy identification (0.2046°), that had visual angle exceed 0.200°.
Suppose we wished to determine a range of minimum visual angles related to the viewing distances; we would begin at a viewing distance of 25 cm, the minimum distance of clear vision for a normal human eye. From here, we would decrease the viewing distances to 20 cm, 15 cm, and 10 cm and increase the viewing distances to 30 cm, 35 cm, and 40 cm. In addition, we would increase the minimum visual angle of 0.200° to 0.250° and 0.300°.
Table 6 displays estimations of the three visual angles’ type size at various viewing distances. At a viewing distance of 25 cm for a normal human eye, the minimum type sizes of the three visual angles should be 0.8727 mm, 1.0908 mm, and 1.3090 mm. At a viewing distance of 20 cm, the type sizes decrease to 0.6981 mm, 0.8727 mm, and 1.0472 mm. However, if we increase the viewing distance to 30 cm, the type sizes increase to 1.0472 mm, 1.3090 mm, and 1.5708 mm. Although it is difficult to define the most appropriate type size for older adults and the visually impaired, using type sizes larger than the type size estimations in Table 6 would benefit all readers.
To assure legibility for a variety of readers, we recommend a minimum Thai type size of 1.3–2 mm Bo Baimai height for reading body text at viewing distances that provide visual angles exceeding 0.200° (see Table 7). In addition, the type size for headlines and subheads should be larger, for example, in the range of 1.5–2.2 mm. This suggestion could conform to the Notification of the Ministry of Public Health (No. 383) (Government Gazette, n.d.). The recommended type sizes vary within the range of 1.3–2 mm and are dependent upon use of typefaces with high legibility and the eyesight differences of readers that determine the range of viewing distances. Moreover, further study of type size may be needed to stipulate requirements for clear and large print documents for Thai readers. The use of small type sizes requires case-by-case evaluation by the drug entrepreneurs based on legibility and readability.
5. Conclusions
In this study we investigated the use of proper typefaces and type sizes on Thai drug packages, patient information leaflets, and medical products through three stages: a literature review of guidelines and regulations, identification of word identification accuracy, and a user preference test. Our results indicate that using illegible typeface and small type sizes may be inappropriate and not serve a variety of readers. These typeface and type size issues are insufficiently specified in Thailand’s guidelines and regulations and require improvement.
To further develop guidelines and regulations based on user-centered care, we suggest using a suitable Thai conventional text typeface for drug labels and documentation. Based on this study’s findings, we recommend consideration of the following typeface characteristics to facilitate the reading of small texts:
- • Presence of distinctive letter features
- • Presence of jutting parts
- • Presence of broader counters in certain letterforms
- • Adequate stroke thickness to support small font accessibility
- • Presence of suitable inter-letter space
- • Avoidance of condensed typefaces
In some cases, it is necessary to use Roman-like Thai typefaces. Drug entrepreneurs and designers should apply careful consideration when using this typeface on drug labels and documentation by utilizing the approach concerning typeface characteristics outlined earlier. We also recommend selecting a suitable Roman-like Thai typeface that includes loop representatives, as stated in our previous study, such as the presence of representative features, jutting parts, and proper inter-letter spacing (Punsongserm & Suvakunta, 2022).
We did not identify a standard type size for legible small text in drug labeling and documentation because this depends on the selected typeface. However, the range of type sizes legible to a variety of readers may be 1.3–2 mm Bo Baimai height for reading body text. The type size for headlines, subheads, and Roman-like Thai typeface should be larger.
Acknowledgments
This work was supported by Thammasat University Research Unit in Social Design.
Notes
Copyright : This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/), which permits unrestricted educational and non-commercial use, provided the original work is properly cited.
References
-
Bastawrous, A., Rono, H. K., Livingstone, I. A., Weiss, H. A., Jordan, S., Kuper, H., & Burton, M. J. (2015). Development and Validation of a Smartphone-Based Visual Acuity Test (Peek Acuity) for Clinical Practice and Community-Based Fieldwork. JAMA Ophthalmol, 133(8), 930-937.
[https://doi.org/10.1001/jamaophthalmol.2015.1468]
-
Beier, S., & Oderkerk, C. (2019). Smaller Visual Angles Show Greater Benefit of Letter Boldness than Larger Visual Angles. Acta Psychologica, 199, 102904.
[https://doi.org/10.1016/j.actpsy.2019.102904]
-
Beier, S., Oderkerk, C., Bay, B., & Larsen, M. (2021). Increased Letter Spacing and Greater Letter Width Improve Reading Acuity in Low Vision Readers. Information Design Journal, 26(1), 1-16.
[https://doi.org/10.1075/idj.19033.bei]
- European Commission. (2009). The Guideline on the Readability of the Labelling and Package Leaflet of Medicinal Products for Human Use. Retrieved June 5, 2022, from https://ec.europa.eu/health/sites/default/files/files/eudralex/vol-2/c/2009_01_12_readability_guideline_final_en.pdf.
- Food and Drug Administration. (2013). Guideline for Leaflet Development. Retrieved August 3, 2022, from https://www.fda.moph.go.th/sites/drug/Shared%20Documents/E-sub/Guideline/4.1%20แนวทางการจัดทำเอกสารกำกับยา.pdf. (In Thai).
- Food and Drug Administration. (2019). Guideline for Leaflet Development for Drug research and Innovation. Retrieved August 3, 2022, from https://www.fda.moph.go.th/sites/oss/Shared%20Documents/SmPC-PIL_HPEP%20guideline_updated%20May2019.pdf.
-
Gluyas, H. (2015). Patient-Centred Care: Improving Healthcare Outcomes. Nursing Standard, 30(4), 50-7.
[https://doi.org/10.7748/ns.30.4.50.e10186]
- Government Gazette. (n.d.). Notification of the Ministry of Public Health (No. 383) B.E. 2560 (2017) Re: Labeling of Food in Containers (No. 2). Retrieved September 9, 2021, from http://www.ratchakitcha.soc.go.th/DATA/PDF/2560/E/097/24.PDF (In Thai).
- Government of Canada. (1999). Guide to the Consumer Packaging and Labelling Act and Regulations. Retrieved June 2, 2022, from https://www.competitionbureau.gc.ca/eic/site/cb-bc.nsf/eng/01248.html.
- Health Products Regulatory Authority. (2022). Guide to Labels and Leaflets of Human Medicines Retrieved July 2, 2022, from https://www.hpra.ie/docs/default-source/publications-forms/guidance-documents/aut-g0034-guide-to-labels-and-leaflets-of-human-medicines-v23.pdf.
- Her Majesty the Queen in Right of Canada. (2016). Good Label and Package Practices Guide for Non-Prescription Drugs and Natural Health Products. Retrieved June 3, 2022, from https://opto.ca/sites/default/files/resources/documents/2016-label-package-practices-pratiques-etiquetage-emballage-non-eng.pdf.
- IC Optix. (2015). Labelling Laws/FDA and EX Guidance. June 5, 2022, from http://www.icoptix.com/vision-impairment/labeling-lawsfda-guidance/.
-
Kawamoto, K., Stanojcic, N., Li, J. P. O., & Thomas, P. B. (2021) Visual Acuity Apps for Rapid Integration in Teleconsultation Services in All Resource Settings: A Review. Asia-Pacific Academy of Ophthalmology, 10(4), 350-354. PMID: 33606386.
[https://doi.org/10.1097/APO.0000000000000384]
-
Leat, S. J., Ahrens, K., Krishnamoorthy, A., Gold, D., & Rojas-Fernandez, C. H. (2014). The legibility of prescription medication labelling in Canada: Moving from pharmacy-centred to patient-centred labels. Canadian Pharmacists Journal/Revue des Pharmaciens du Canada, 147(3), 179-187. PMID: 24847371; PMCID: PMC4025884.
[https://doi.org/10.1177/1715163514530094]
-
Minakata, K. & Beier S. (2021). The Effect of Font Width on Eye Movements During Reading. Applied ergonomics, 97, 103523.
[https://doi.org/10.1016/j.apergo.2021.103523]
- Mitchell, B. (2014). On Loops and Latinisation. Retrieved July 29, 2022, from http://www.fontpad.co.uk/loops-and-latinisation/.
- National Patient Safety Agency. (2007). Design for Patient Safety: A Guide to the Graphic Design of Medication Packaging. Retrieved June 5, 2022, from https://silo.tips/download/a-guide-to-the-graphic-design-of-medication-packaging.
- Obama, T., Ikeda, M., Sagawa, K., & Shinoda, H. (2005). Range of similar colours with and without cataract experiencing goggles. in AIC 2005, Proceedings of the 10th Congress of the International Color Association. Granada: International Colour Association (AIC).
-
Oderkerk, C. A., & Beier, S. (2022). Fonts of wider letter shapes improve letter recognition in parafovea and periphery. Ergonomics, 65(5), 753-761.
[https://doi.org/10.1080/00140139.2021.1991001]
- Office of the Council of State. (2019). Drug Act (No. 5), B.E. 2530. Retrieved September 9, 2021, from http://web.krisdika.go.th/data/law/law2/%C204/%C204-20-2530-005.htm (in Thai).
- Panasonic Corporation. (2021). Panasonic Universal Design Book. Retrieved July 15, 2022, from https:// https://www.panasonic.com/jp/corporate/technology-design/ud/pdf/udbook_2021.pdf.
- Phuangsuwan, C., & Ikeda, M. (2017). Indoor Illuminance to Provide Elderly Cataract People with Proper Visual Performance. Journal of Ratchasuda College for Research and Development of Persons with Disabilities, 13(1), 56-67.
- Punsongserm, R. (2019). Thai Universal Design Font Versus Familiar Thai Text Fonts: The Role of Distinctive Letterforms and Suitable Inter-letter Space Influence in Blurred Words. In Proceeding of Heritage & Vision: The 2019 International Conference on Design for Experience and Wellbeing (143-202). Xi'an: Northwestern Polytechnical University.
-
Punsongserm, R. (2020). Comparative Effectiveness of Homologous Thai Letterforms on Visual Word Recognition: Thai Universal Design Font Versus Familiar Thai Text Fonts. Archives of Design Research, 33(3), 19-43.
[https://doi.org/10.15187/adr.2020.08.33.3.19]
-
Punsongserm, R., & Suvakunta, P. (2022). Do the Small Thai Font Sizes on Drug Labels and Documentation Facilitate Thai Readers? A Practical Review. Archives of Design Research, 35(1), 51-73.
[https://doi.org/10.15187/adr.2022.02.35.1.51]
-
Punsongserm. R., Sunaga, S., & Ihara, H. (2017a). Thai Typefaces (Part 1): Assumption on Visibility and Legibility Problems. Archives of Design Research, 30(1), 5-23.
[https://doi.org/10.15187/adr.2017.02.30.1.5]
-
Punsongserm, R., Sunaga, S., & Ihara, H. (2017b). Thai Typefaces (Part 2): Criticism Based on Legibility Test of Some Isolated Characters. Archives of Design Research, 30(2), 23-45.
[https://doi.org/10.15187/adr.2017.05.30.2.23]
-
Punsongserm, R., Sunaga, S., & Ihara, H. (2018a). Effectiveness of the Homologous Thai Letterforms on Visibility under a Simulated Condition of Low Visual Acuity. In Proceeding of Annual Conference of the 11th Typography Day, Mumbai: Industrial Design Centre (IDC) and Indian Institute of Technology Bombay (IIT Bombay).
[https://doi.org/10.1075/idj.00002.pun]
-
Punsongserm, R., Sunaga, S., & Ihara, H. (2018b). Effectiveness of Homologous Thai Letterforms Presented in Parafoveal Vision. Information Design Journal, 24(2), 92-115.
[https://doi.org/10.1075/idj.00002.pun]
- Punsongserm, R., Sunaga, S., & Ihara, H. (2018c). Roman-Like Thai Typefaces: Breakthrough or Regression? In Proceeding of Conference of ICDHS 10th + 1: Back to the Future/The Future in the Past (580-585). Barcelona: Universitat de Barcelona.
- Rattanakasamsuk, K. (2013). Elderly Vision on Legibility of Thai Letters Presented on LED Panal. ACA 2013 Thanyaburi: Blooming Color for Life (70-73). Thanyaburi: Asia Color Association and Rajamangala University of Technology Thanyaburi.
- Rosal, M. (2020). Rating Scales in UX Research: Likert or Semantic Differential? Retrieved June 5, 2022, from https://www.nngroup.com/articles/rating-scales/.
- Santayayon, M., Pipitpukdee, J., & Phantachat, W. (2011). A Study of the Legibility of Thai Letters in Thai Young Adults Aged 19-25 Years Old and Older Adults Aged 60 Years Old and Over. In Proceedings of the 5th International Conference on Rehabilitation Engineering & Assistive Technology. Bangkok: Swissotel Nai Lert Park.
- Swissmedic. (2022). Guidance Document: Packaging for Human Medicinal Products HMV4. Retrieved June 7, 2022, from https://www.swissmedic.ch/dam/swissmedic/en/dokumente/zulassung/zl_hmv_iv/zl000_00_021d_wl_wegleitung_angaben_auf_packmittel.pdf.download.pdf/ZL000_00_021e_WL%20Guidance%20document%20Information%20on%20packaging.pdf.
- The Federal Agency for Medicines and Health Products. (2020). Labelling of Medicinal Products. Retrieved June 3, 2022, from https://www.famhp.be/sites/default/files/content/POST/MAH/163-en-labelling_of_medicinal_products.pdf.
- The Institute for Safe Medication Practices Canada. (2018). Good Label and Package Practices Guide for Non-Prescription Drugs and Natural Health Products. Retrieved June 6, 2022, from https://www.canada.ca/content/dam/hc-sc/documents/services/drugs-health-products/reports-publications/medeffect-canada/good-label-package-practices-guide-non-prescription-drugs-natural-health-products-guidance/good-label-package-practices-guide-non-prescription-drugs-natural-health-products-eng.pdf.
- The Institute for Safe Medication Practices Canada. (2019). Good Label and Package Practices Guide for Prescription Drugs. Retrieved June 5, 2022, from https://publications.gc.ca/collections/collection_2019/sc-hc/H164-195-1-2019-eng.pdf.
- U.S. Department of Health and Human Services. (2013). Guidance for Industry: Labeling for Human Prescription Drug and Biological Products-Implementing the PLR Content and Format Requirements Retrieved July 30, 2022, from https://www.fda.gov/media/71836/download.
- U.S. Department of Health and Human Services. (2018). Guidance for Industry Labeling OTC Human Drug Products. Retrieved July 30, 2022, from https://www.fda.gov/media/72441/download.
- Usakunwathana, P. (2015). The Second Decade of Sukhumvit-Sukhumvit Tadmai. Retrieved July 29, 2022, from http://anuthin.org/2015/01/12/ทศวรรษที่สองของสุขุมวิ/. (In Thai).
- Waleetorncheepsawat, B., Pungrassamee, P., Ikeda, M., & Obama, T. (2013). Proper-sized Thai Letters on Different Background Contrasts and Illumination Environment Suitable for Elderlies. In Proceedings of ACA2013 Thanyaburi: Blooming Color for Life (74-77). Thanyaburi: Asia Color Association and Rajamangala University of Technology Thanyaburi.
- Wilkinson, I. (2005). Inclusive Design: Clear and Large Print Best Practice Guide for Designers. Taunton: International Society of Typographic Designers.
-
Wongsompipatana, P., Ikeda, M., & Katemake, P. (2011). Equivalent Lightness of Elderlies Investigated by Cataract-Experiencing Goggles. Color Research & Application, 38(4), 267-276.
[https://doi.org/10.1002/col.20737]